In Vivo Oncology
Testing the efficacy of a new drug candidate in mouse models is a crucial step towards the clinical phase of drug development. The jump of complexity from cellular models to animal testing is huge even when using more complex cellular 3D models to prepare for the in vivo phase.
Before testing the drug’s efficacy in mice, our team will have performed several studies in regards to ADME, the maximum-tolerated dose, and pre-formulation experiments.
Reaction Biology’s animal facility is located in Germany and certified according to ISO 9001. We provide tumor models for efficacy testing in xenograft and immuno-oncology models.
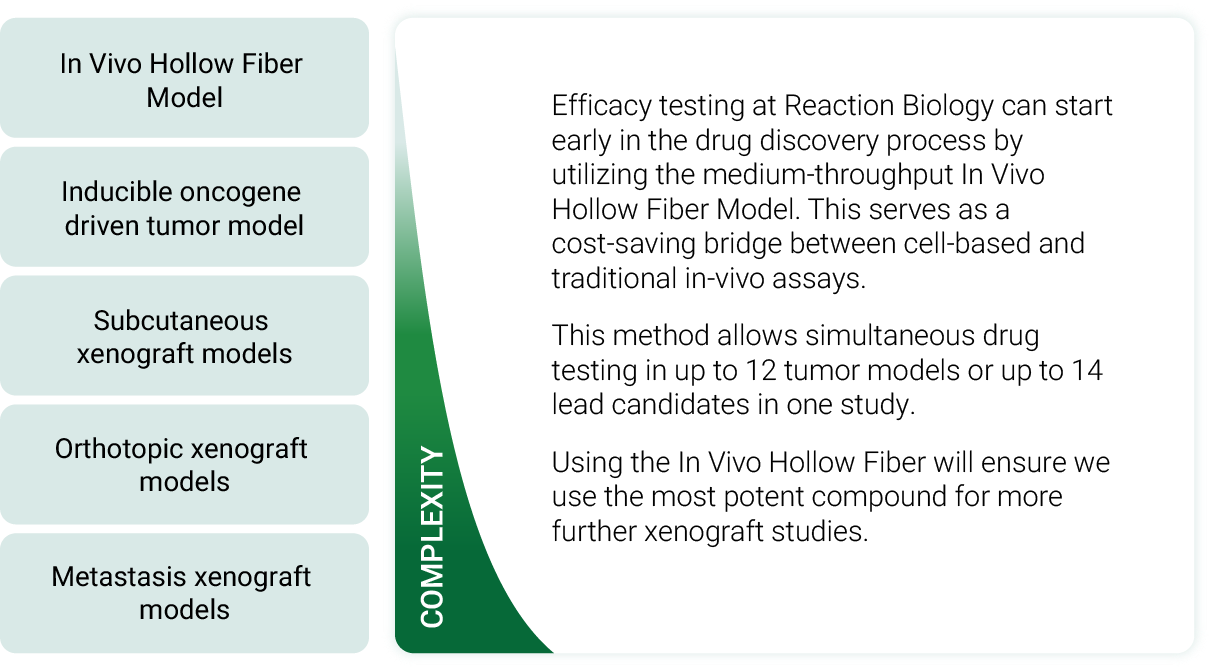
Medium-throughput screening via the In Vivo Hollow Fiber Model
The integration of the Hollow Fiber Model in the compound screening workflow allows the selection of:
a. the most efficacious compound
b. the most susceptible tumor model
Genetically engineered models tailored to examine your target in vivo
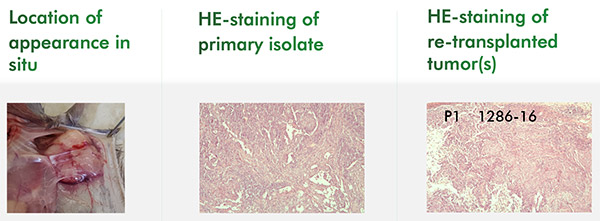
Reaction Biology can test the efficacy of drugs in genetically engineered models. To this end, fibroblasts are stably transfected with a proliferation-driving oncogene (the target) which than can be inhibited in the mouse. The testing is performed in fully immunocompetent mice. Such models serve as excellent readouts of a single driver of tumor growth, such as a constitutively active kinase.
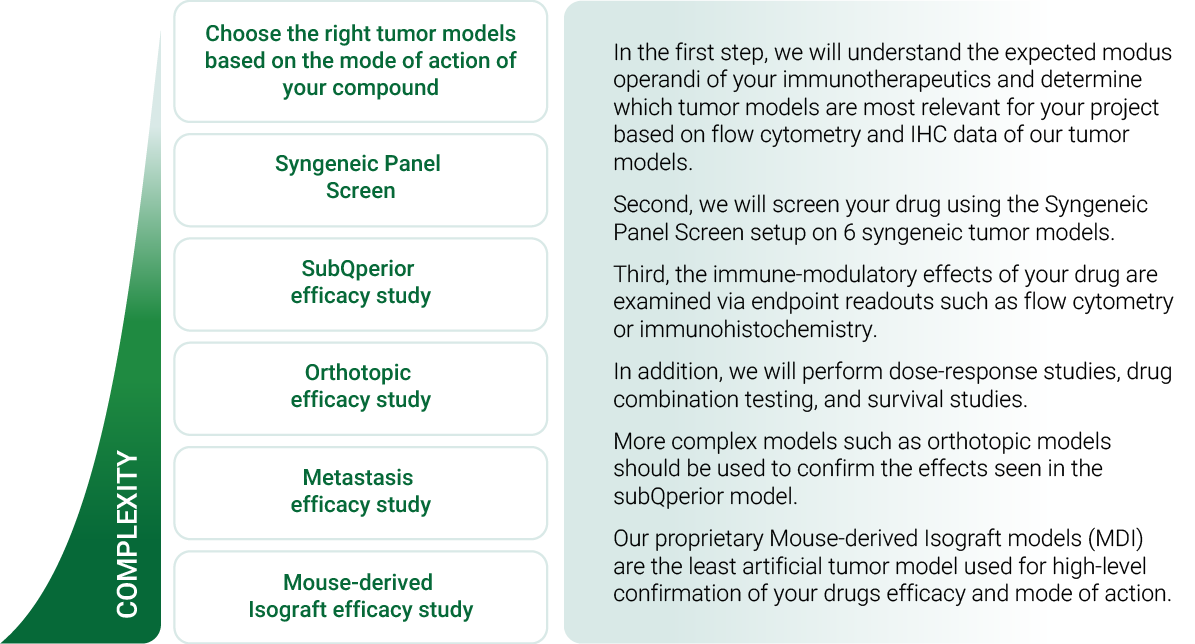
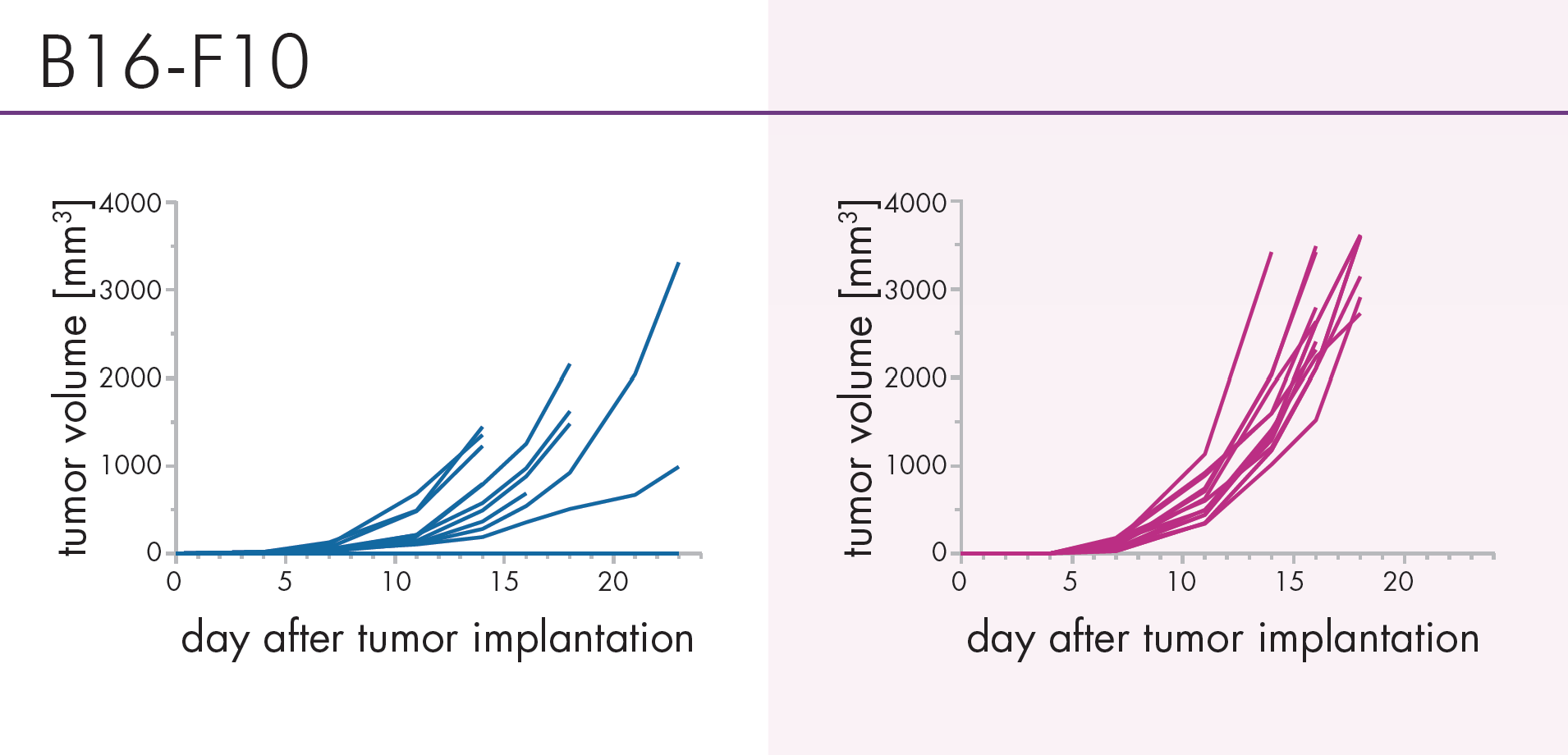
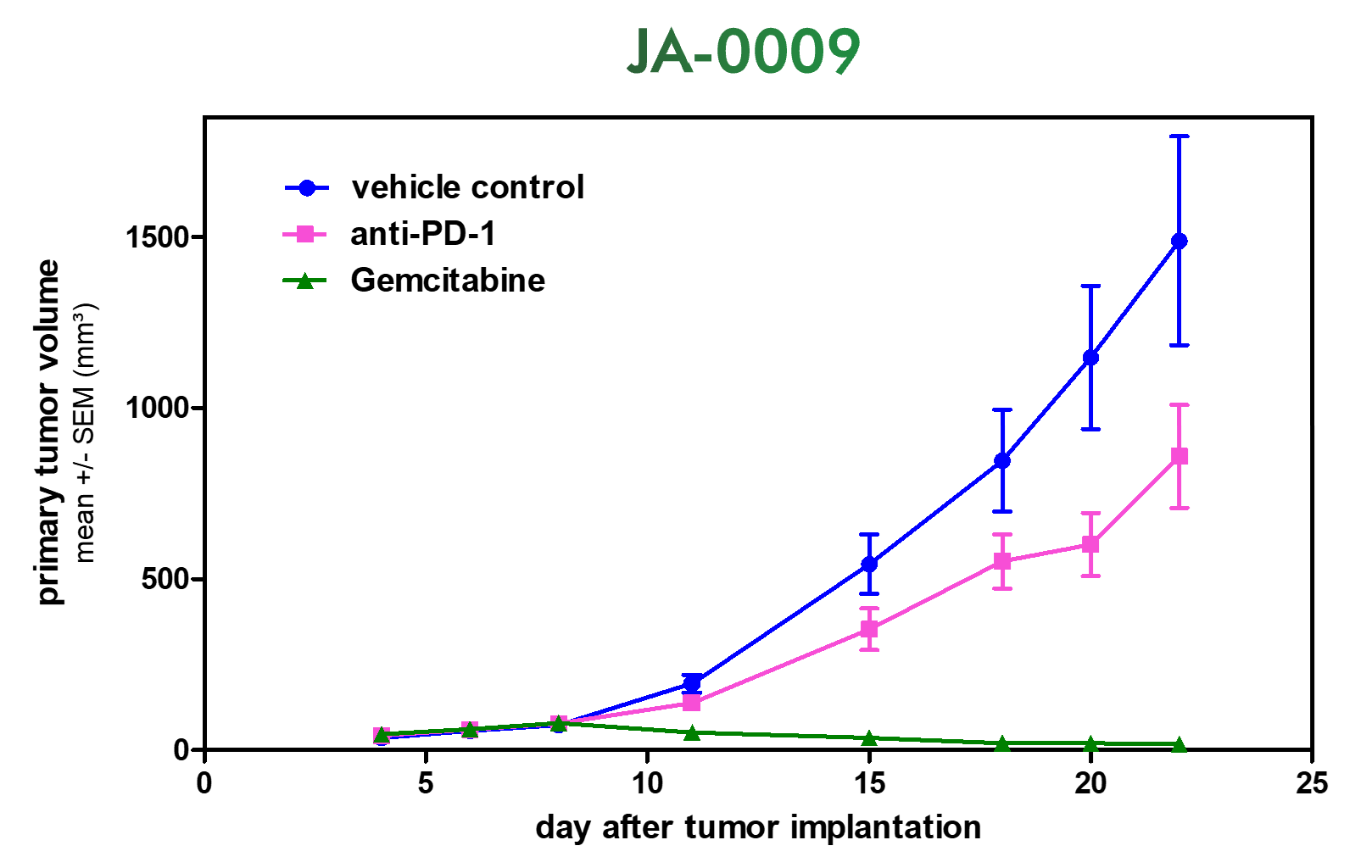
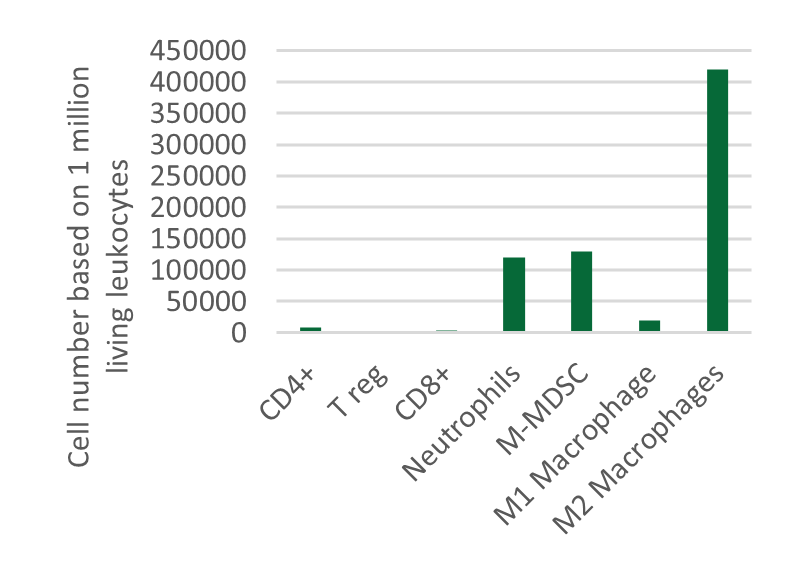
An Innovative Immunooncology Model Suite
Testing new candidates for immunotherapy needs careful determination of the goals and selection of suitable tumor models.
Immunotherapy often results in best responses when combined either with immune-checkpoint inhibitors and/or with conventional therapeutics.
Therefore, testing the immune-modulatory effects after therapy may yield the wanted results, even when a reduction in tumor size cannot be accomplished.
Our team of scientists has many years of experience in the immuno-oncology space and will guide your project towards meaningful testing of your new immunotherapeutics. See below our immunotherapy testing workflow.