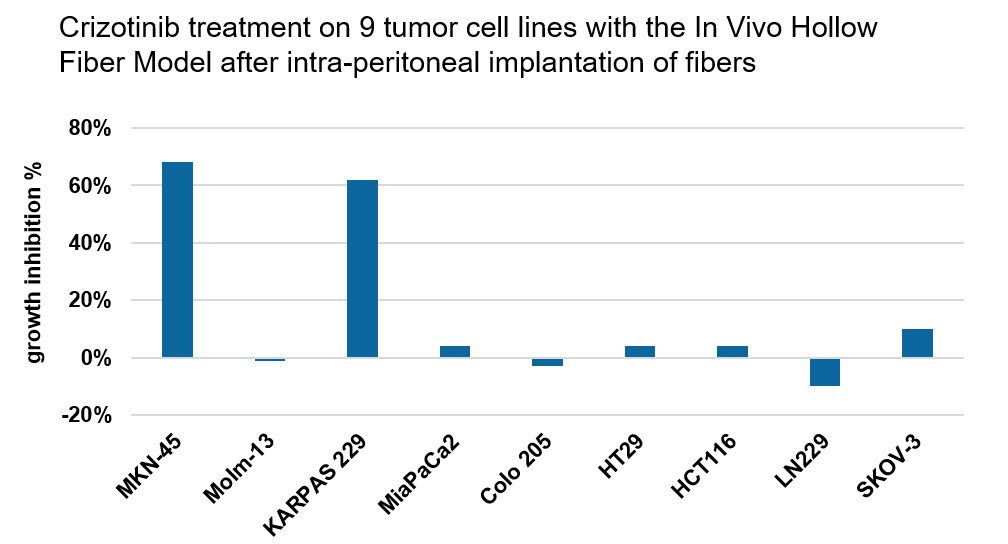
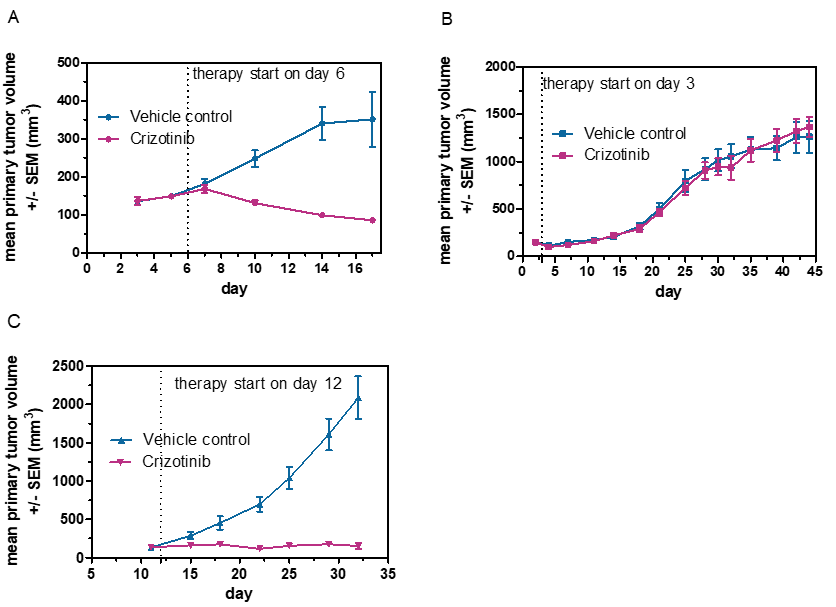
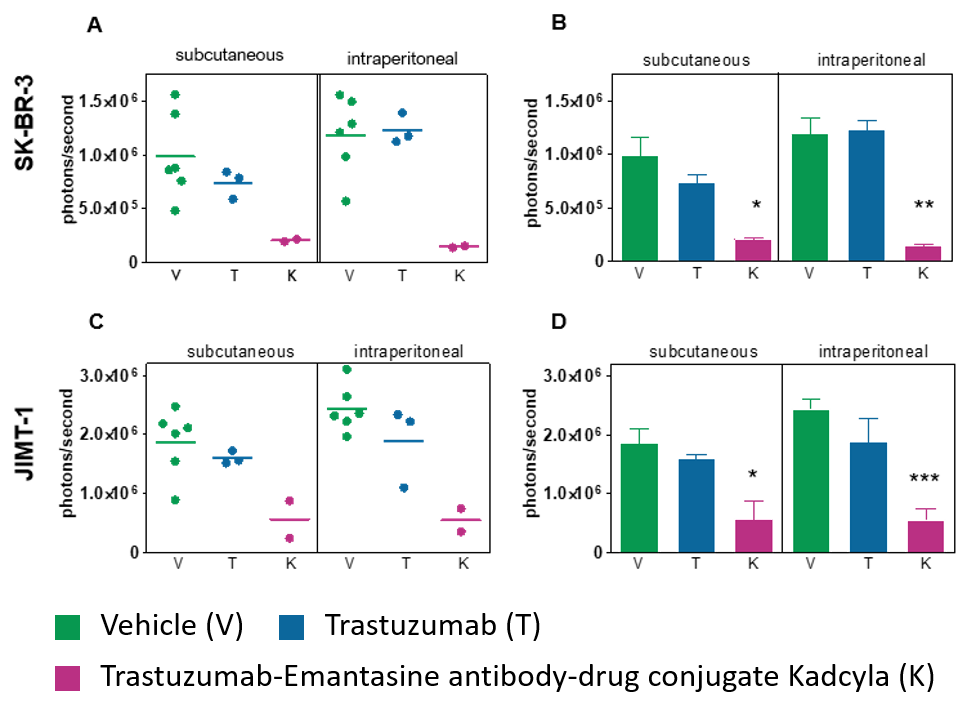
In Vivo Hollow Fiber Model
The In Vivo Hollow Fiber Model is a drug screening assay bridging the gap between in vitro and in vivo testing of anti-cancer agents allowing for rapid answers regarding drug efficacy.
The method is based on growing three tumor cell lines into one mouse, allowing a fast and economic screening approach with the following advantages:
- Use the Hollow Fiber Model to select the best drug candidates and tumor models for subsequent xenograft tumor testing with higher predictability than conventional assays.
- Any human tumor cell line can be included in a study. We can provide tumor cell lines of our collection of 253 cell lines or use a custom cell line for testing.
- The Hollow Fiber Model can be performed early in the drug discovery process with simultaneous evaluation of pharmacokinetic and pharmacodynamic parameters.