3D Tumor Spheroid Assay Service
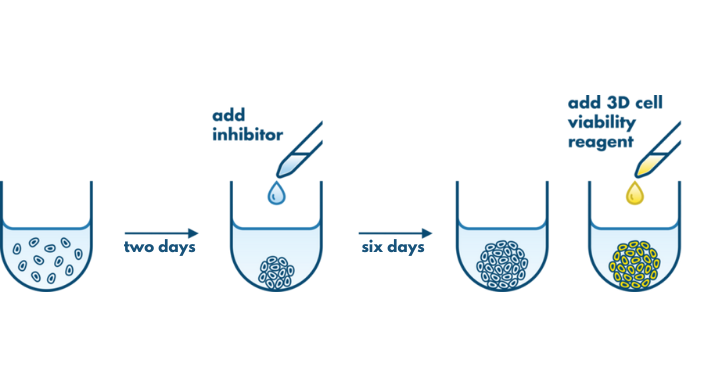
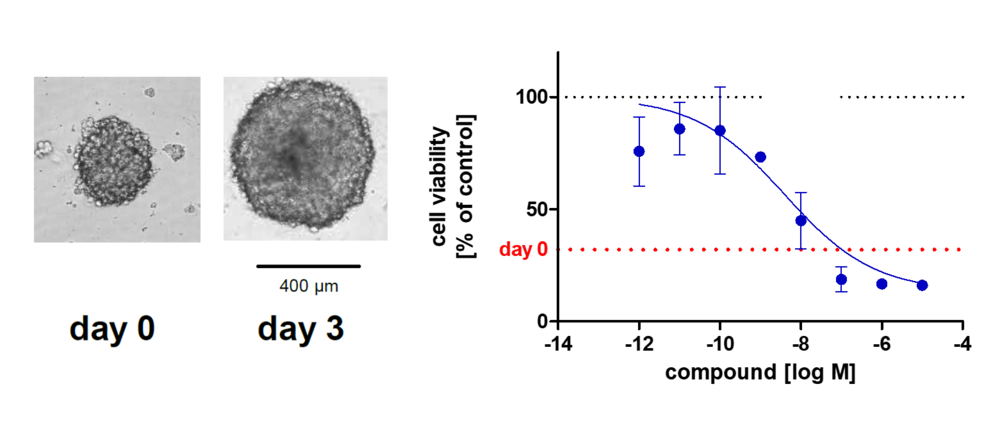
The 3D Tumor Spheroid Assay is used to analyze the therapeutic effect and tumor-penetrating capacity of anti-cancer therapeutics. This 3D assay is performed on spheroids with high translational value making it a valuable tool for lead candidate selection for in vivo studies.
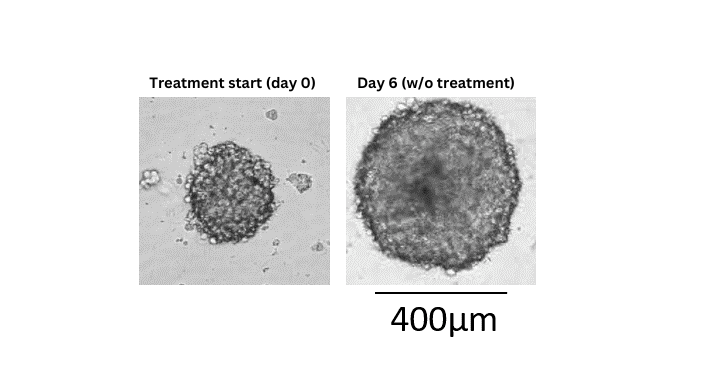
3D tumor spheroids recapitulate some of the complex processes that compounds face in an in vivo situation such as cellular barriers and cells adapted to gradients of oxygen and nutrient concentrations. The spheroids are composed of cells in various proliferative and metabolic states similar to tumors. Therefore, the 3D Tumor Spheroid Assay has a high predictive value for pre-clinical drug research.
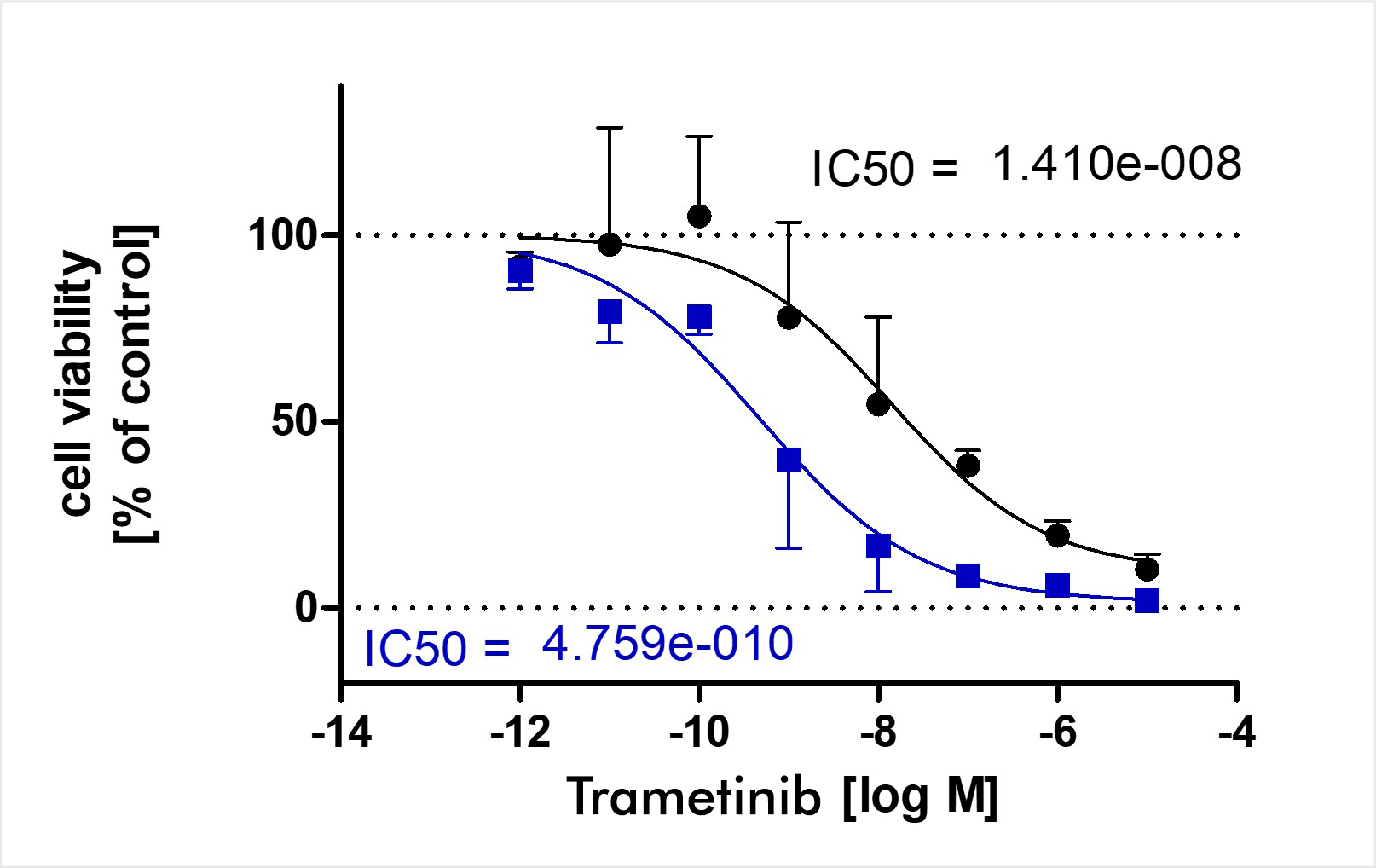
The 3D Tumor Spheroid Assay is one of two 3D assays offered by Reaction Biology. The 3D Tumor Spheroid Assay is used for testing a drug’s anti-proliferative and cytotoxic capacity on existing tumor spheroids. In the Soft Agar Assay, which is also a 3D assay, we test the impact of drugs on the proliferation of single cells growing into colonies.
- Mono-spheroid assay with human tumor cell lines
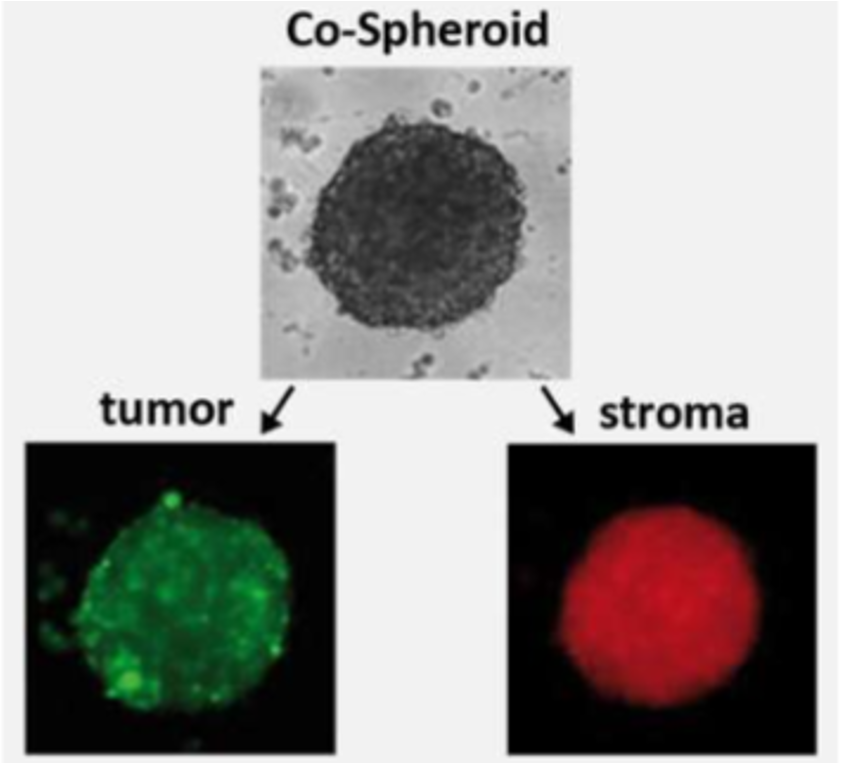
- Co-culture spheroid testing with quantification of both, stroma and tumor cells
- High-throughput compatible setup