ADME-Tox
The integrated approach of the Reaction Oncology Platform provides testing of ADME-tox parameters (Adsorption, Distribution, Metabolism, Excretion, and Toxicity) throughout the whole drug discovery process.
Reaction Biology offers a basic suite of ADME assays in-house, such as:
- CYP enzyme inhibition assays
- In vivo PK/PD studies in mice
- Plasma Inhibitory Assay to define the impact of plasma proteins on the function of new drug candidates
- Safety panel to test off-target effects on a selection of 40 GPCR, ion channels, transporters, and enzymes whose inhibition may cause toxic side effects.
To provide our clients a complete portfolio of ADME-tox services, we work with a team of specialists from the high-quality ADME-tox provider Admescope. Partnering with an experienced ADME-tox CRO ensures the creation of drug candidates with a favorable pharmacologic profile necessary for approval by regulatory bodies. Admescope is a part of our medicinal chemistry partner Symeres, aiding well-tuned communication routines and logistics for short cycle times to realize optimal drug discovery.
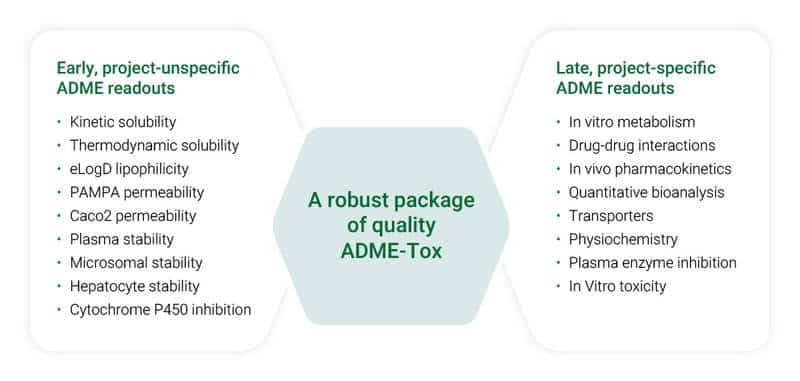
With the integrated ADME-Tox capabilities, we support the project in the hit-to-lead, lead optimization, and preclinical development phase. The tailorable services are widely spread over the whole nonclinical ADME-Tox area: in vitro & in vivo drug metabolism; including metabolite profiling & identification, drug-drug interactions, pharmacokinetics on different rodent species, and quantitative bioanalysis.
With an eye on the regulatory requirements for successful IND application, we integrate ADME readouts into the drug discovery process to ensure:
- To only advance drugs with a favorable pharmacological profile.
- Hone the drug candidates early in the process to ensure high bioavailability in the organism.
- Determine drug-drug interactions, in vitro toxicity, and inhibition of metabolic enzymes to ensure low side effects.

