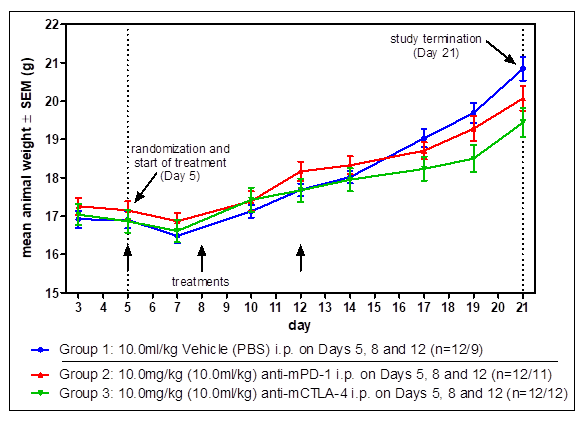
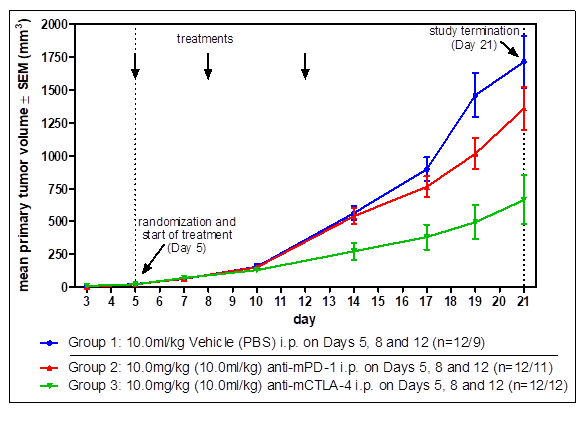
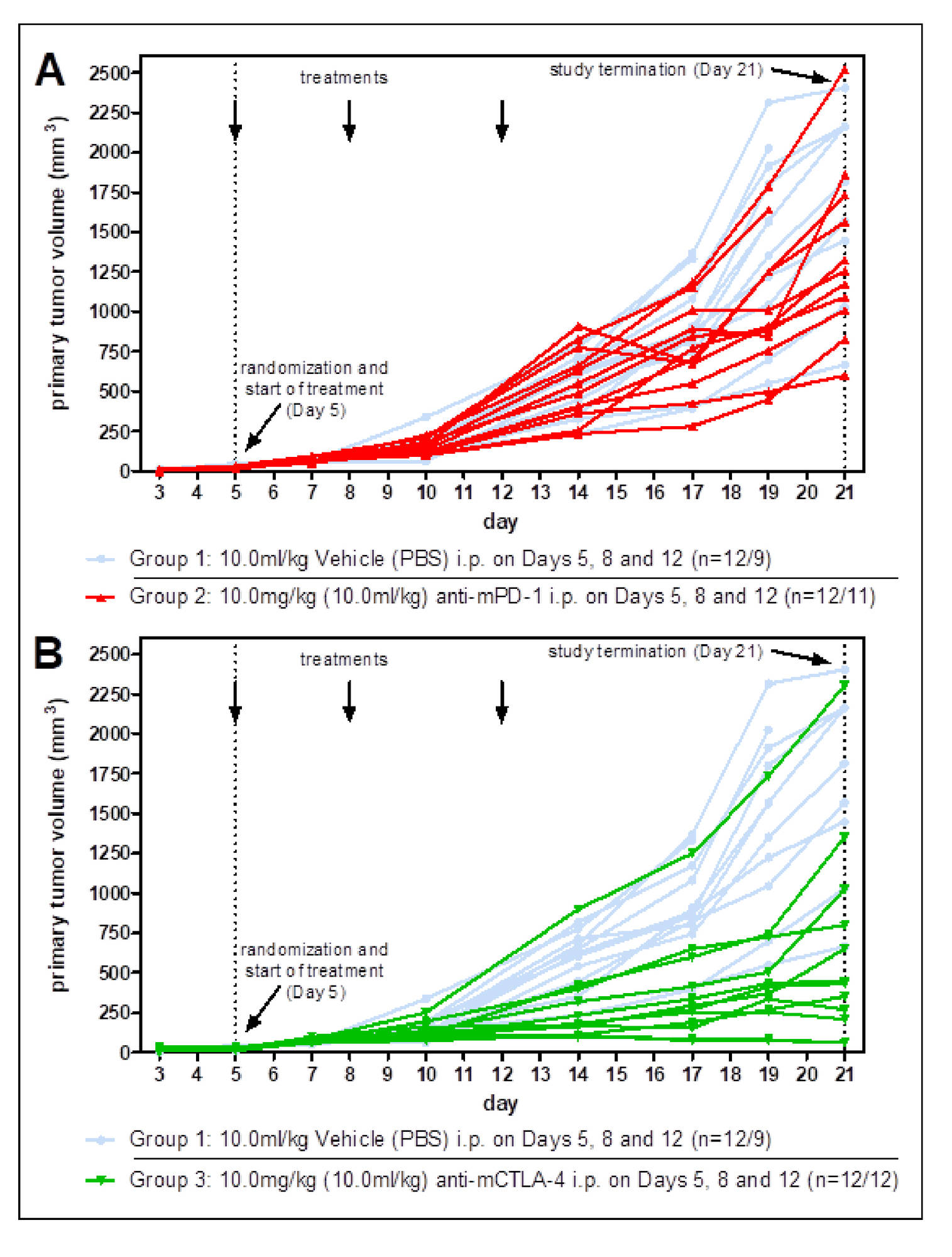
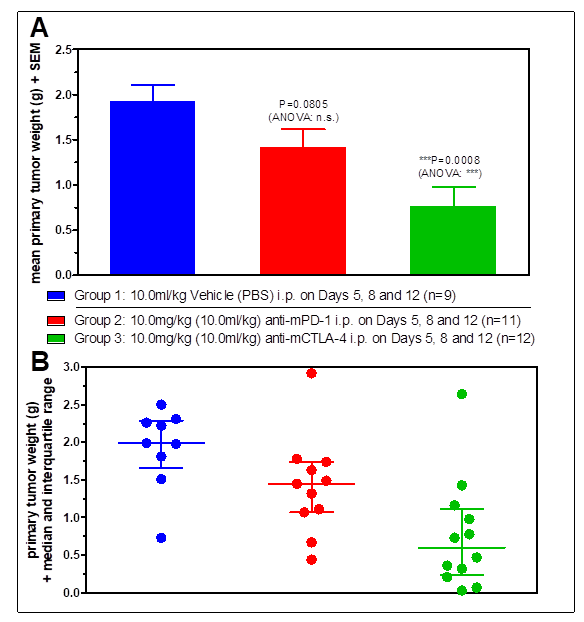
Standard Efficacy Studies: Determination of the therapeutic effect of a new drug on a syngeneic tumor model.
Panel Screening: On a regular basis, Reaction Biology performs panel screenings for the determination of therapeutic efficacy on 6 syngeneic tumor models. This economic screening option includes vehicle as well as anti-PD1 treatment arms – free of charge.
Proof-of-Concept Studies: New drug candidates need to be tested not only for efficacy but also for their mechanism of action in the animal. For example, exploratory proof of concept studies can be performed via flow cytometric analysis for investigation of effects on tumor-infiltrating immune cells.
Pharmacokinetic Studies: Pharmacokinetic studies on tumor-bearing mice are helpful in determining the concentration of drugs not just in plasma but in the tissue of the tumors.
Pharmacodynamic Studies: Pharmacodynamic studies elucidate whether and how a drug acts on its target for example, via flow cytometric analysis of tumor-infiltrating immune cells.
Dose-Response-Relationship: Determine suitable drug doses for efficacy testing.
Drug Combination Studies: Combinations of drugs can lead to synergistic effects and vastly increased tumor response. Generally, we validate our syngeneic tumor models with immune-checkpoint inhibitors anti-PD1, anti-PDL1, and anti-CTLA4, which are often used for combination therapy.
Survival Studies: A survival study setup is used to obtain information about a long-term effect of the test substance in comparison to control. Survival studies give meaningful results for ambivalent drug candidates measuring the number of responders and non-responders.