Historically, orthotopic models had to be sacrificed at multiple time points to evaluate the tumor load. Thanks to advances in molecular biology and optical imaging, it has become possible to track the tumor progression within the animal in real-time.
Once implanted into the bladder, whole body luminescent images are overlayed on photographic images to follow tumor growth. This approach not only reduces animal use and associated costs, but also enables animals to be more accurately randomized to treatment arms, and tumor tracking can be performed non-invasively without animal termination.
We have successfully transduced 11 different clones from the MB49 BC cell line with that we have used to establish a collection of orthotopic bladder cancer models with different growth characteristics and immune profiles. To maintain the highest quality of data, each cancer cell clone used to establish the orthotopic model is validated for growth and luminescence in vitro and is checked for in vivo luminescence over time and terminal tumor weight.
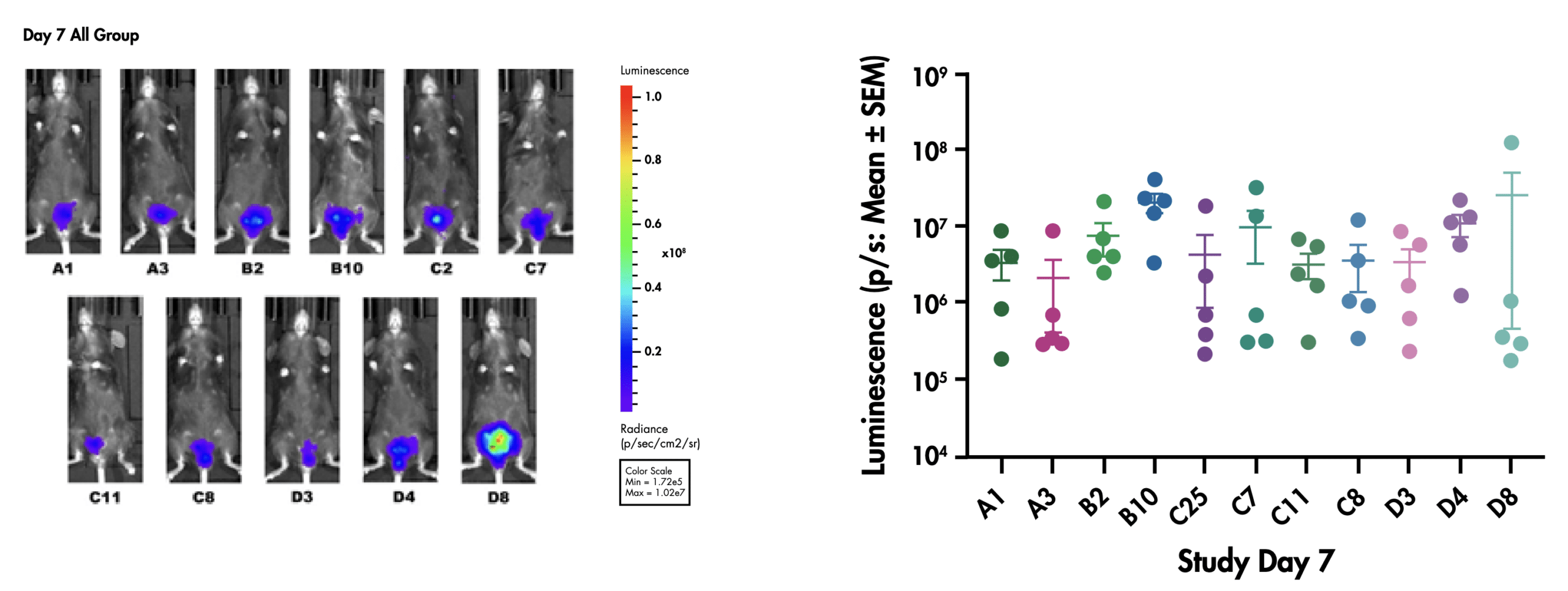
Figure: Representative in vivo luminescent signal in different clones of NMIBC bladder wall instillation models (left) Average luminescence for different clones (n=5) 7 days post inoculation (right).
Moreover, our flow cytometry analysis for measuring TILs infiltration and T cell proliferation has identified differences between immune cell infiltration between tumor clones allowing you to select from hotter or colder tumor models for downstream analysis. Our comprehensive approach ensures that you’re equipped with the best tools to advance your bladder cancer research and develop superior treatments.

Figure: Characterization of MB49_luc clones used to establish NMIBC bladder wall instillation model. This data shows the analysis of tumor-infiltrating lymphocytes (TILs) and T cell proliferation profile using flow cytometry.
Our standard service extends beyond in vivo luminescence data. In addition to terminal tumor weight measurements, we offer a robust program of clinical observations throughout the study. The observation data can provide valuable insights that complement the bioluminescent data and aid in a more complete understanding of the effect of your clinical candidate on tumor burden and overall animal health.
Furthermore, we understand the critical role of regulatory compliance in drug development. Our team of experienced medical writers can translate your research findings into clear, concise, and IND-ready study reports. These reports meet all the necessary requirements set forth by regulatory agencies, ensuring a smooth path towards clinical trials and ultimately, new treatment options for bladder cancer patients.





