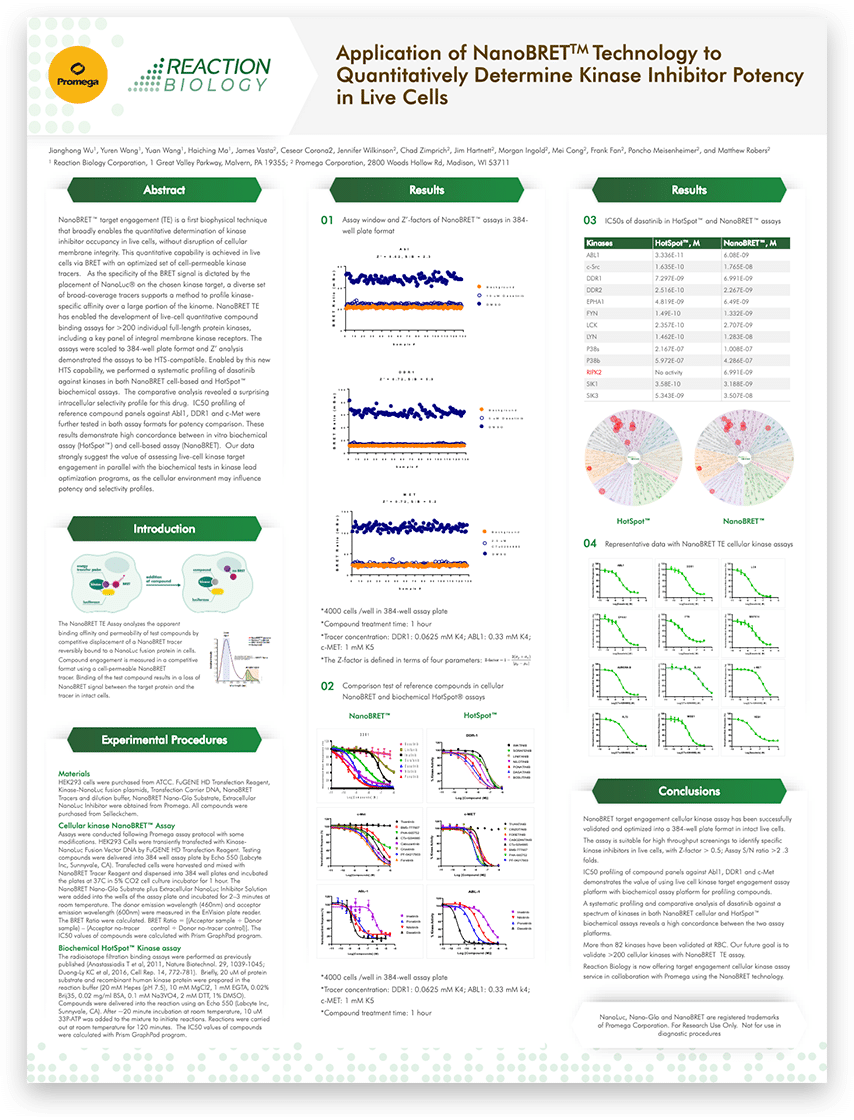
Live and Direct: How NanoBRET™ Enables Real-Time Kinase Activity Detection
Inspired by nature’s bioluminescence, NanoBRET™ offers a powerful tool for studying Kinase activity in live cells. This blog explores the advantages of NanoBRET for kinase target engagement and its role in drug discovery. Read further to learn its principle of working and its application in kinase research.
The concept of Bioluminescence Resonance Energy Transfer draws its inspiration directly from nature. In the jellyfish Aequorea victoria, bioluminescence is orchestrated through the interaction of aequorin and green fluorescent protein (GFP). Aequorin, a calcium-binding protein, emits a blue glow when it binds with calcium ions (Ca²⁺). This blue light is then transferred to GFP via resonance energy transfer. GFP absorbs the blue light and emits a striking green bioluminescence. This energy transfer from an excited donor fluorophore to an acceptor fluorophore was later defined as Bioluminescence Resonance Energy Transfer (BRET). The first lab-led BRET experiment, conducted in 1999, studied protein-protein interactions between circadian clock genes in cyanobacteria, using Renilla luciferase (Rluc) as the energy donor and yellow fluorescent protein (YFP) as the acceptor in both in vitro and in vivo systems.

Learn from our whitepaper Exploring kinase inhibitor selectivity and affinity in live cells
History of Advances in BRET
Building on the foundational BRET technology of the late 1990s, NanoBRET™ emerged, propelled by the development of NanoLuc luciferase. NanoLuc, a small (19kD) bright luciferase derived from deep-sea shrimp, achieved substantial improvements in sensitivity and dynamic range over conventional BRET methods, due to greater spectral separation between the donor and acceptor, as well as higher donor light intensity. This innovative method quickly gained traction for studying protein interactions and compound binding with precision, leading to applications in drug discovery, high-throughput screening, and various protein families.
NanoBRET™ for Kinase Target Engagement
NanoBRET™ technology is essential for studying kinase target engagement (TE). It has already been applied to evaluate ALK4/ALK5, SIK2/SIK3 and cyclin-dependent kinase inhibitors. The process can be broken down into following steps:
- Expression of Kinase-NanoLuc Fusion: The kinase of interest is expressed in mammalian cells as a fusion with NanoLuc luciferase.
- Design of NanoBRET™ Tracer: A cell-permeable fluorescent NanoBRET™ tracer is created by tagging a compound of interest, typically a known inhibitor, with a fluorescent dye.
- Addition of Tracer to Cells: The fluorescent NanoBRET™ tracer is introduced into the cells
- Addition of test compounds: Unlabeled test compounds are introduced to the cells. These compounds bind to the target kinase if they have an affinity for it.
- Addition of Substrate and Inhibitor: Following the tracer, a substrate and an extracellular inhibitor are added to the cells.
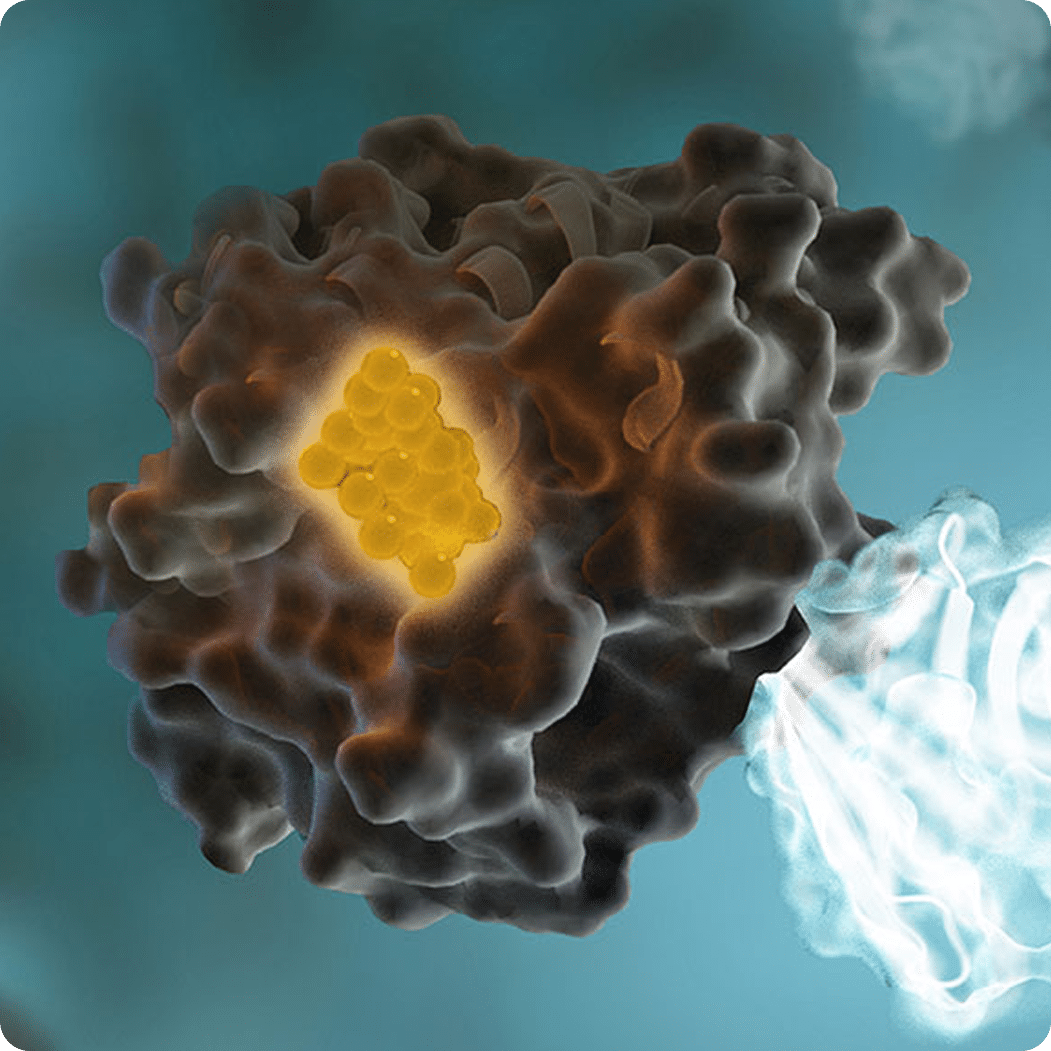
- Generation of BRET Signal: The expression of the kinase-NanoLuc fusion protein facilitates a strong BRET signal between the kinase-NanoLuc protein and the NanoBRET™ tracer. This occurs because the NanoLuc substrate reacts with NanoLuc luciferase, producing light that is transferred to the fluorescent dye on the tracer if in close proximity.
- Ensuring Signal Specificity: The extracellular NanoLuc inhibitor ensures that the BRET signal is derived from live, uncompromised cells by blocking any extracellular NanoLuc activity, preventing non-specific luminescence.
- Measurement of BRET Signal: The BRET signal is measured. If the test compounds bind to the target kinase, they displace the NanoBRET™ tracer, resulting in a loss of the BRET signal.

Due to BRET’s stringent distance constraints, the data obtained are specific to the kinase fused with NanoLuc luciferase. This allows for accurate quantification of kinase-compound interactions, making NanoBRET™ technology a valuable tool in kinase research and drug discovery.
Key Advantages of NanoBRET™ for Kinase Activity Detection
The key advantages of NanoBRET™ for kinase activity detection include:
- Live-cell measurements: Unlike many traditional kinase assays that require cell lysis, NanoBRET™ allows for real-time monitoring of kinase activity in intact cells. This preserves the physiological context and provides more biologically relevant data.
- High sensitivity: The brightness of NanoLuc and the optimized energy transfer make NanoBRET™ extremely sensitive, enabling detection of interactions even at low expression levels of the target kinase.
See the science behind it:
Application of NanoBRET™ technology to quantitatively determine kinase inhibitor potency in live cells
- Quantitative binding data: NanoBRET™ provides quantitative information on compound binding affinities and kinetics within cells. This allows researchers to determine IC50 values and residence times for inhibitors in a cellular context. Broad applicability: The technique has been successfully applied to various kinase families, including tyrosine kinases, serine/threonine kinases, and lipid kinases.
- Selectivity profiling: NanoBRET™ enables comprehensive selectivity profiling of compounds against large panels of kinases in live cells. This is crucial for assessing off-target effects and optimizing drug candidates.
- Compatibility with high-throughput screening: The assay can be miniaturized and automated, making it suitable for screening large compound libraries.
NOW ENROLLING!
NanoBRET™ Intracellular CDK Target Engagement Screening
Outlook & Conclusion
This technology continues to evolve, with recent developments focusing on streamlining the assay workflow. A modified protocol now allows the use of a single kinase tracer for profiling compound binding against a panel of 192 kinases, greatly simplifying large-scale selectivity studies. Moreover, researchers are integrating it with other technologies like DNA-encoded libraries (DELs) for probe development. Additionally, they are exploring applications of the technology in other protein classes and cellular processes. NanoBRET™ continues to evolve, solidifying its role as a crucial technique in kinase drug discovery and beyond.
References
- Xu Y, Piston DW, Johnson CH. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):151-6. doi: 10.1073/pnas.96.1.151. PMID: 9874787; PMCID: PMC15108.
- De A, Jasani A, Arora R, Gambhir SS. Evolution of BRET Biosensors from Live Cell to Tissue-Scale In vivo Imaging. Front Endocrinol (Lausanne). 2013 Sep 23;4:131. doi: 10.3389/fendo.2013.00131. PMID: 24065957; PMCID: PMC3779814.
- Machleidt T, Woodroofe CC, Schwinn MK, Méndez J, Robers MB, Zimmerman K, Otto P, Daniels DL, Kirkland TA, Wood KV. NanoBRET–A Novel BRET Platform for the Analysis of Protein-Protein Interactions. ACS Chem Biol. 2015 Aug 21;10(8):1797-804. doi: 10.1021/acschembio.5b00143. Epub 2015 Jun 9. PMID: 26006698.