Antibody-drug conjugates (ADCs) have emerged as a revolutionary approach to cancer therapy. These “smart bombs” combine the targeting power of monoclonal antibodies with the potent killing ability of cytotoxic drugs. Unlike traditional chemotherapy, which harms both healthy and cancerous cells, ADCs are designed to deliver their deadly payload directly to tumors, minimizing side effects. This blog post explores the exciting potential of ADCs while also delving into the significant challenges hindering their development.
Unravelling the promise of ADCs: Download our free factsheet
Before you dive into the exciting world of ADCs, equip yourself with the knowledge to navigate their development process. Download our free factsheet.
ADCs: The Magic Bullet
ADCs are composed of three key components: the monoclonal antibody (mAb), which specifically targets an antigen on cancer cells; the linker, which connects the mAb to the cytotoxic drug and ensures targeted delivery; and the cytotoxic payload, a drug that disrupts vital processes inside the cancer cell, leading to cell death.
For an ADC to be effective, several critical steps must occur:
- Selective binding: The mAb must precisely recognize and bind to the cancer antigen, avoiding healthy tissues.
- Internalization: The entire ADC-antigen complex needs to be engulfed by the cancer cell.
- Drug release: Inside the cell, the linker must break down, releasing the cytotoxic drug to do its job.
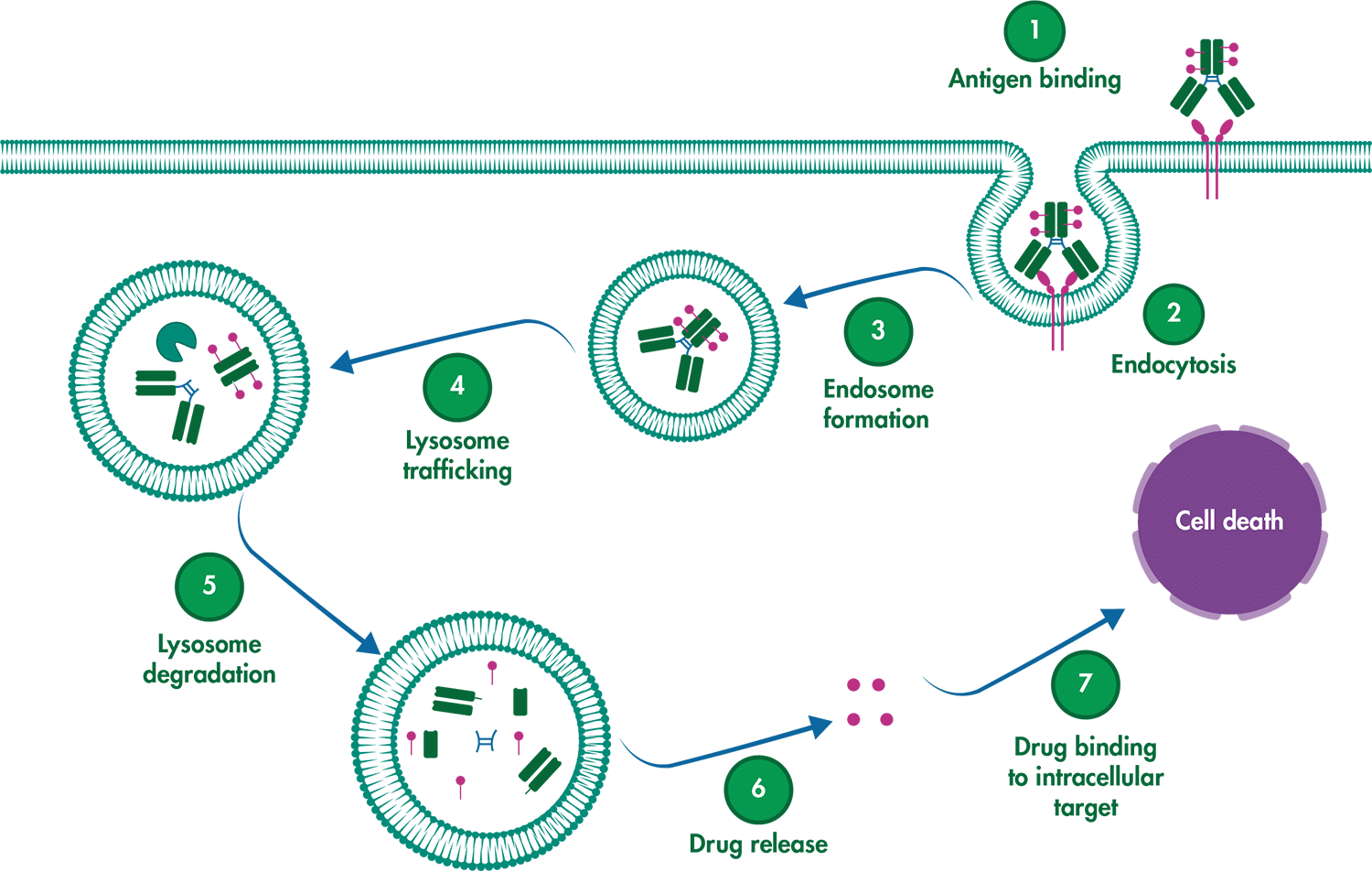
Schematic depicting the mechanism of action of an ADC
Balancing Act: Ensuring Safety and Efficacy
Developing safe and effective ADCs requires a delicate balance. One key challenge is maintaining a controlled drug-to-antibody ratio. Too high a ratio can lead to the drug detaching prematurely and harming healthy cells (bystander effect). Conversely, a low ratio might not deliver enough punch to kill cancer cells.
The past four decades have witnessed significant strides in ADC development. Scientists have engineered more specific mAbs, improved linker stability, and fine-tuned drug-to-antibody ratios. However, the path to success is paved with setbacks. Despite a growing list of approved ADCs and 150+ ongoing clinical trials, a concerning number fail to reach the market.
Why do ADCs Fail?
According to a detailed literature review of ADC clinical landscape published in August 2023 1, the primary culprit for the discontinuation of 92 ADC trials to date is insufficient efficacy at tolerable doses. Simply put, many ADCs aren’t potent enough to kill cancer cells at safe dosages. This often leads to dose-limiting toxicities, severe side effects that force researchers to reduce the dose, effectively rendering the treatment ineffective. These toxicities often occur in healthy tissues, highlighting the difficulty of achieving true target specificity. Moreover, some payloads seem to be inherently toxic, causing problems regardless of the target antigen 2.
Elevate your ADC antigen discovery with OncoFlow-Profiler
Test the expression of your target antigen with our rapid and high-throughput platform
The Road Ahead
Despite the challenges, the potential of ADCs remains undeniable. Researchers are actively exploring strategies to overcome these hurdles, including
- Developing next-generation linkers that are more stable and allow for targeted drug release within the tumor.
- Engineering even more specific mAbs to minimize off-target effects.
- Identifying novel cytotoxic payloads with improved potency and reduced toxicity.
Overcome ADC development hurdles with our end-to-end service portfolio
We offer a comprehensive suite of services to support you at every step of the ADC development process:
- Antigen binding specificity
- Internalization kinetics analysis
- Payload cytotoxicity profiling
- In vivo studies for tolerability and safety
- GxP potency assays
Conclusion
ADCs represent a powerful weapon in the fight against cancer. While significant challenges remain, ongoing research holds immense promise for the future of this innovative therapy. By optimizing design strategies and addressing safety concerns, ADCs have the potential to revolutionize cancer treatment, offering patients a more precise and effective approach to battling this devastating disease.
Resources
- Maecker H, Jonnalagadda V, Bhakta S, Jammalamadaka V, Junutula JR. Exploration of the antibody-drug conjugate clinical landscape. MAbs. 2023 Jan-Dec;15(1):2229101
- Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers (Basel). 2023 Jan 24;15(3):713. doi: 10.3390/cancers15030713
- Holtorf et al., Development and evaluation of a high-throughput method for rapid detection of surface antigen expression in fixed cells. In: Proceedings of the Annual Meeting of the American Association for Cancer Research; 2024 Apr 1–5; San Diego (CA): AACR; 2024. Abstract nr 5157.



