Macrophages are phagocytic cells that eliminate pathogens and modulate adaptive immunity through cytokine secretion. However, within the TME, they exhibit phenotypic plasticity, adopting either an anti-tumor M1 or pro-tumor M2 phenotype depending on environmental cues. M1 macrophages promote anti-tumor immunity, while M2 macrophages support tumor growth and immunosuppression.
Targeting TAMs: Redefining the Therapeutic Landscape
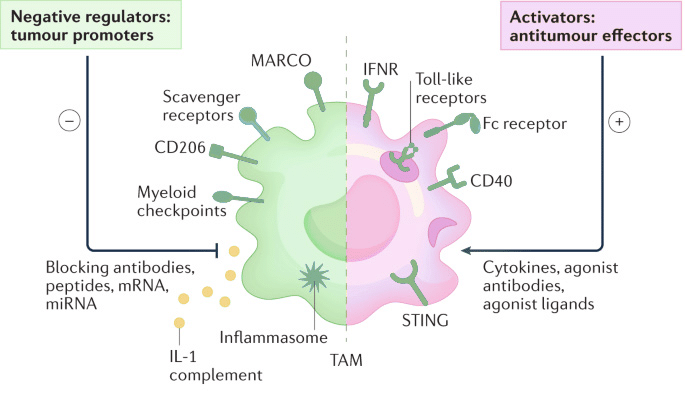
Tumor-associated macrophages (TAMs) are a dense population of M2 macrophages in the TME that create an immunosuppressive shield. Drug developers are currently focusing on the following strategies for the development of these next-generation immunotherapies:
- Reprogram TAMs towards an anti-tumor M1 phenotype.
- Activate TAMs to unleash their intrinsic tumor-killing potential.
- Disrupt TAM-mediated immunosuppression.
- Modulate TAM metabolism for therapeutic benefit.
- Utilize cellular therapies like CAR-macrophages for targeted tumor elimination.
The burgeoning field of macrophage-targeted therapies necessitates robust tools for evaluating their efficacy. Reaction Biology addresses this need with a comprehensive suite of four assays designed to assess macrophage function within the TME:
1. Macrophage Polarization Assay:
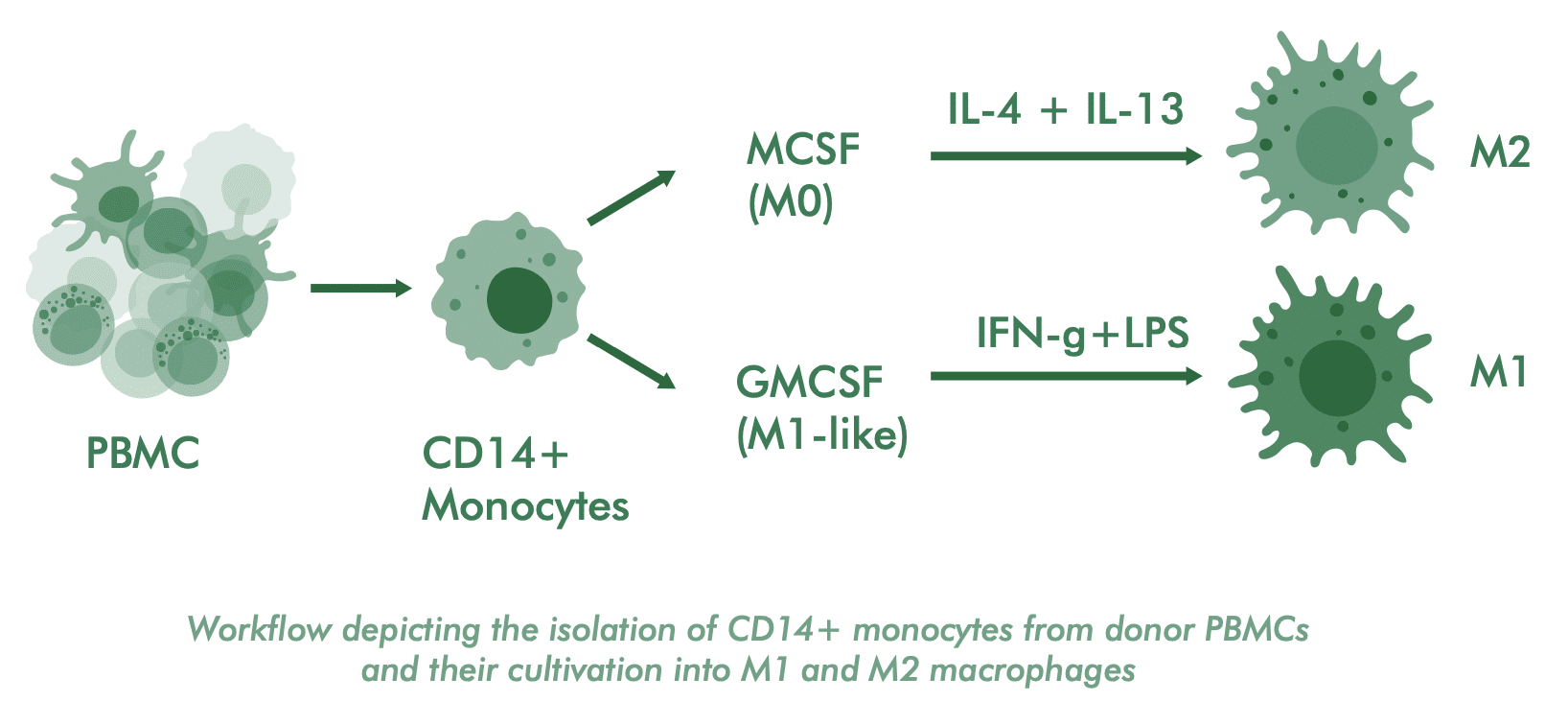
This assay quantifies how your immunotherapeutic modulates macrophage differentiation. Isolated CD14+ monocytes from healthy donors are differentiated into various subtypes under defined conditions. Flow cytometry measures the impact on surface marker expression (CD86, CD163, CD206) to identify specific macrophage subsets. Additionally, cytokine profiling further complements the characterization of the induced functional phenotype.
2. Phagocytosis Assay:
This assay is designed to test the phagocytic capabilities of macrophage subtypes or monocytes using fluorescently labelled bioparticles. The principle of the assay lies in the assessment of phagocytic activity in digesting these bioparticles which could be detected by flow cytometry or real-time measurements using microscopy.
3. Monocyte Killing Assay:
This assay measures the therapeutic’s ability to induce monocyte-mediated cytotoxicity and inflammatory cytokine release. CD14+ monocytes co-cultured with luciferase-expressing target cells are exposed to the immunotherapeutic. Cell viability measurements determine monocyte killing activity, while cytokine release quantification confirms monocyte activation.
4. Multiplex Cytokine Assay:
This assay quantifies cytokine secretion triggered by immunotherapeutic activation. Isolated CD14+ monocytes co-cultured with luciferase-expressing target cells are stimulated. Following activation, supernatants are collected and analyzed using MSD multiplex assay to identify specific secreted cytokines.
Macrophages represent a promising therapeutic target in the ever-expanding field of cancer immunotherapy. By understanding how to manipulate their polarization, phagocytosis, and cytokine secretion, researchers can develop effective strategies to overcome the immunosuppressive TME and unleash the full potential of the immune system against cancer. Reaction Biology’s suite of macrophage assays provides researchers with the essential tools to dissect these complex interactions and pave the way for the next generation of macrophage-based immunotherapies.


