Testing the efficacy of lead compounds in animal models is a crucial step in preclinical drug development. Researchers have many in vivo tumor models to choose from, but selecting the right one involves considering factors such as cost, time, and technical expertise. Additionally, it’s important to assess whether the model provides an immune-competent environment for pharmacokinetic/pharmacodynamic (PK/PD) studies, supports robust tumor growth, and allows for longer treatment windows to generate statistically significant data.
ON-DEMAND WEBINAR
Listen to our experts explain SubQperior™ Technology
One key factor affecting all these considerations is the tumor implantation method. Traditional subcutaneous implantation, though common, poses significant challenges, including a high risk of ulceration that leads to premature study termination, shortened treatment windows, and increased variability in tumor growth. These issues often reduce statistical reliability and compromise the significance of study outcomes.
SubQperior™ addresses these challenges head-on, offering a superior approach that not only enhances tumor growth and stability but also provides consistent and reliable data. Here’s why SubQperior™ stands out.
| Aspect |
SubQperior™ |
Traditional Subcutaneous |
Genetically Engineered Models (GEMM) |
Human Xenograft Models |
| Tumor Growth |
Homogeneous and consistent growth |
Often heterogeneous with variable growth |
Typically homogeneous |
Often variable, reflects human tumor behaviour |
| Study Window |
Long window with minimal ulceration |
Shortened due to ulceration |
Long window, ulceration not applicable |
Long window, ulceration not applicable |
| Cost |
Cost-effective |
Generally low cost |
Expensive |
High cost due to complexity and maintenance |
| Ease of setup |
Simple to set up |
Simple to set up |
Complex with specific genetic modifications |
Requires specialized skills and resources |
| Data Consistency |
Consistent and reproducible |
Variable due to tumor growth issues |
Reliable but dependent on genetic background |
High-variability; human-specific responses |
Table comparing different in vivo models for immuno-oncology research
Robust Tumor Growth & Improved Statistical Power
SubQperior™ uses mammary fat pad injection, creating a stroma-rich environment that acts as a buffer between the tumor and dermis. This reduces ulceration and supports more homogeneous tumor growth, which reduces variability and enhances the statistical power of your studies.
Longer Treatment Windows
In traditional subcutaneous models, ulceration is the main cause for study termination. SubQperior™ technology overcomes ulceration as the cause of termination. Along with the technology supporting robust tumor growth, it enables longer treatment windows.
Let the Data Reveal:
SubQperior™ vs. Conventional Subcutaneous Models
Check out our whitepaper to learn how SubQperior™ excels in tumor growth, treatment window, immune cell infiltration, and much more.
Optimized Resources & Cost-Effective
Another advantage of SubQperior™ is its ability to optimize the use of research resources. The consistent growth of tumors in these models means fewer mice are needed per treatment arm, reducing the overall number of animals required for your studies. This lowers costs and aligns with ethical guidelines by minimizing animal subject use. With SubQperior™, you’re not just getting superior results—you’re doing so in a more efficient and responsible way. Moreover, Reaction Biology’s SubQperior™ Panel Screen offers exceptional value by providing data from three treatment arms—including free anti-PD1 and control arms.
SubQperior™ Panel Screen
Triple the data, Double the Value. Pay for one arm, get data from three, including anti-PD1 and control groups, free.
Comparable Immune Response
SubQperior™ models offer more than just homogeneous tumor growth conditions; they also feature higher immune cell infiltration compared to traditional subcutaneous models. Immune Checkpoint Inhibitor (ICI) treatments produce similar responses in both SubQperior™ and subcutaneous models, maintaining the relevance of your therapeutic studies. Furthermore, SubQperior™ models exhibit a range of immune phenotypes and display more extensive immune cell infiltration, providing a more detailed platform for evaluating the immune landscape in cancer.
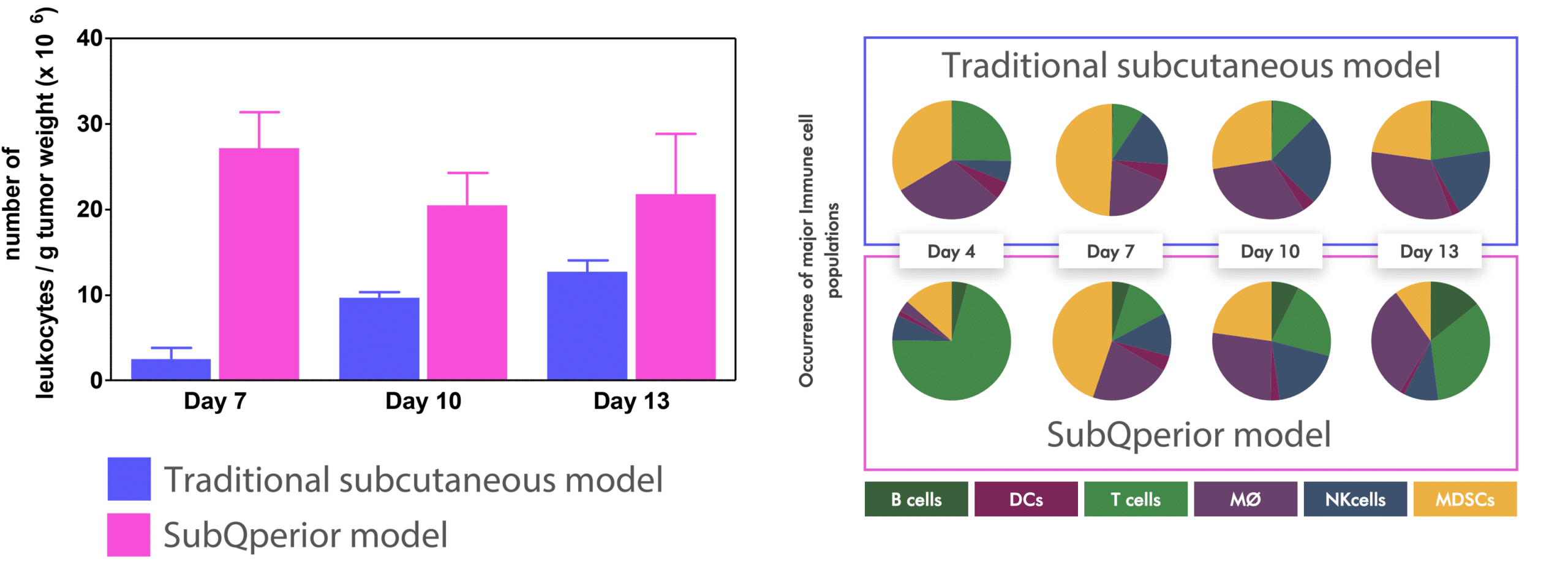
SubQperior™ models demonstrate higher leukocyte levels on day 13 post-tumor implantation compared to conventional subcutaneous models.
Conclusion
SubQperior™ overcomes the challenges of traditional subcutaneous implantation, offering a superior alternative that enhances tumor growth, improves data reliability, mirrors immune responses, optimizes resources, and delivers exceptional value.



